Titan's Lakes and Seas May Be Fizzy With Patches of Nitrogen Bubbles
Such bubble formations may also explain the mystery "magic island" phenomenon.
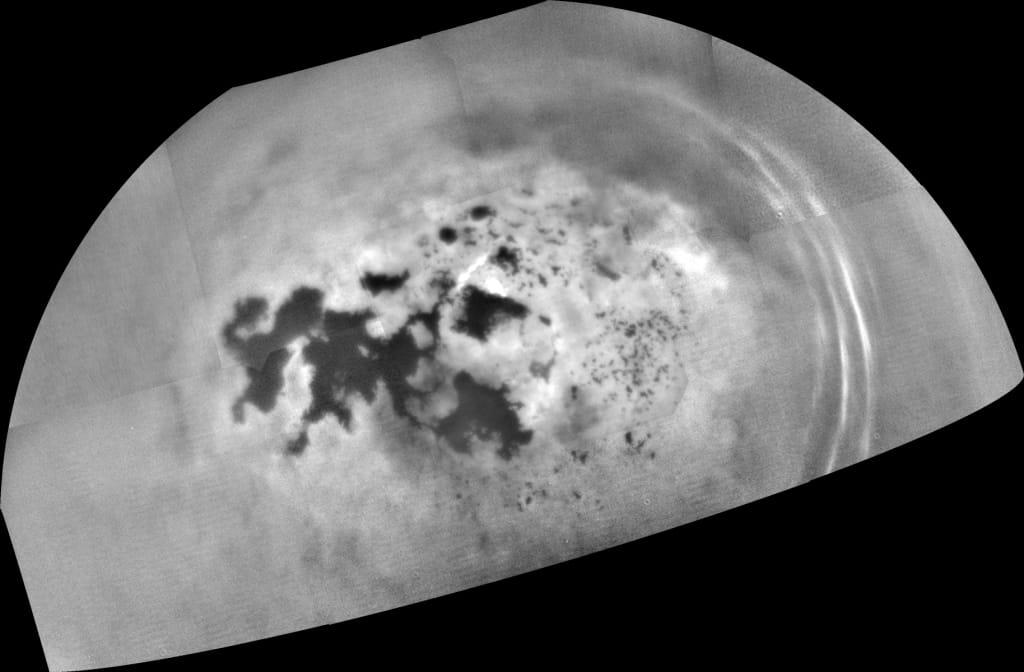
Saturn's moon Titan is the only other body in the Solar System besides Earth known to have liquids on its surface. In Titan's case, they are rivers, lakes and seas of liquid hydrocarbons (methane/ethane) instead of water. There is even methane/ethane rain, which further mimics Earth's hydrological cycle. For the most part, the lakes and seas are fairly smooth, with only small amounts of wave activity. But now, new research suggests that those lakes and seas might be quite fizzy at times - with periodic bursts of nitrogen bubbles.
In the new study, researchers simulated the conditions on Titan - it is extremely cold, which is why methane and ethane play the role of water. On Titan, water ice is as hard as rock. The study showed that significant amounts of nitrogen can be dissolved in the cold liquid methane/ethane. When there are slight changes in temperature, air pressure or composition, the nitrogen can rapidly separate out of solution, similar to the fizz that results when opening a bottle of carbonated soda.
The new results have been published online in the journal Icarus. From the abstract:
"We obtained laboratory measurements of nitrogen solubility in mixed solutions of ethane and methane at temperatures and pressures relevant to Titan's lakes and seas. Our results show that nitrogen solubility is increased at higher methane concentration, lower temperature, and higher pressure. We developed an empirical fit that agrees well with our measurements. We show that significant volumes of nitrogen gas will be released from Titan lake fluids on heating, and that significant volumes of nitrogen gas will be absorbed by Titan lake fluids on cooling. The densities of the lake fluids will be affected by nitrogen dissolution. We also show that mixing of two cryogenic fluids of different composition can lead to the release of large amounts of nitrogen gas. This has implications for lake fluids, bubble formation, geological phenomena, and also for future landed missions on the surface of Titan. We find in particular that methane-rich lakes at lower temperatures on Titan will be the most sensitive to changes in surface conditions, either cooling, heating, or compositional mixing."
The composition of the lakes and seas can vary from place to place, with some having more methane and others having more ethane.
"Our experiments showed that when methane-rich liquids mix with ethane-rich ones - for example from a heavy rain, or when runoff from a methane river mixes into an ethane-rich lake - the nitrogen is less able to stay in solution," said Michael Malaska of JPL, who led the study.
In the small simulated Titan lake, the researchers were able to coax nitrogen out of a simulated ethane-rich solution as the ethane froze on the bottom. Ethane ice formed on the bottom of the lake, and as the ethane crystalizes into ice, there's no room left for the dissolved nitrogen gas, so it comes fizzing out on the surface of the lake.
Fizzy bubbles may also help to explain another mystery on the Titan - the so-called "magic islands" where small patches in the seas have been observed to appear and disappear. Various explanations have been proposed, including fields of bubbles.
"Thanks to this work on nitrogen's solubility, we're now confident that bubbles could indeed form in the seas, and in fact may be more abundant than we'd expected," said Jason Hofgartner of JPL, who is a co-investigator on Cassini's radar team and is a co-author of the new study.
'Magic island' feature in Ligeia Mare
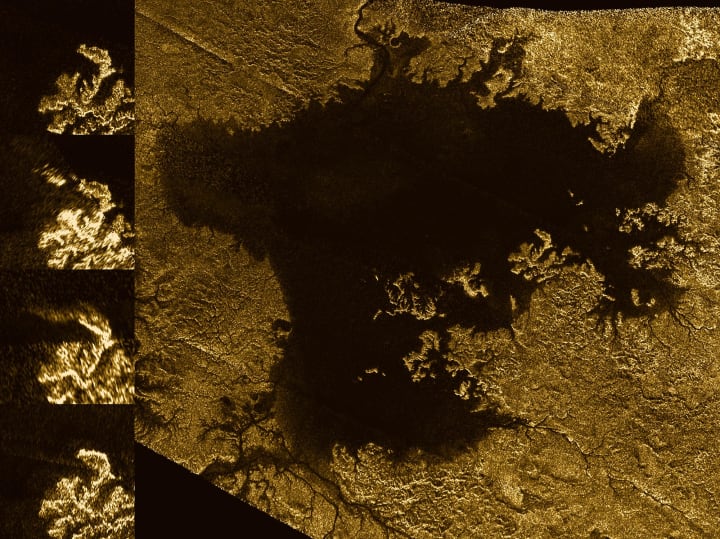
Radar image from Cassini showing the large hydrocarbon sea named Ligeia Mare on Titan, and the evolution of a "magic island" feature (inset side panel). Image by NASA/JPL-Caltech/ASI/Cornell
"In effect, it's as though the lakes of Titan breathe nitrogen," Malaska said. "As they cool, they can absorb more of the gas, 'inhaling.' And as they warm, the liquid's capacity is reduced, so they 'exhale.'"
On Earth, something similar happens when carbon dioxide is absorbed by the oceans.
On April 22, 2017, the Cassini spacecraft will conduct its last close flyby of the mission (with 127 flybys in total). Cassini's radar beam will again sweep over the lakes and seas in the northern hemisphere. Scientists are hoping that if any more "magic islands" are seen, they will be better able to distinguish between bubbles, waves and floating or suspended solids as the explanation.
After the Titan flyby, Cassini will begin its last series of 22 dives through the gap between Saturn and its innermost rings. This part of the mission is known as The Grand Finale - on Sept. 15, 2017, Cassini will plunge into Saturn's atmosphere to meet its planned fate after the probe's fuel finally runs out. It will be a dramatic end to an incredible mission which has revolutionized our knowledge of the Saturn system.
More information about the Cassini mission is available here.
About the Creator
Paul Scott Anderson
Paul is a freelance space writer and blogger who currently writes for AmericaSpace and Vocal. His own blog Planetaria is a chronicle of planetary exploration.
paulscottanderson.ca
planetaria.ca






Comments
There are no comments for this story
Be the first to respond and start the conversation.